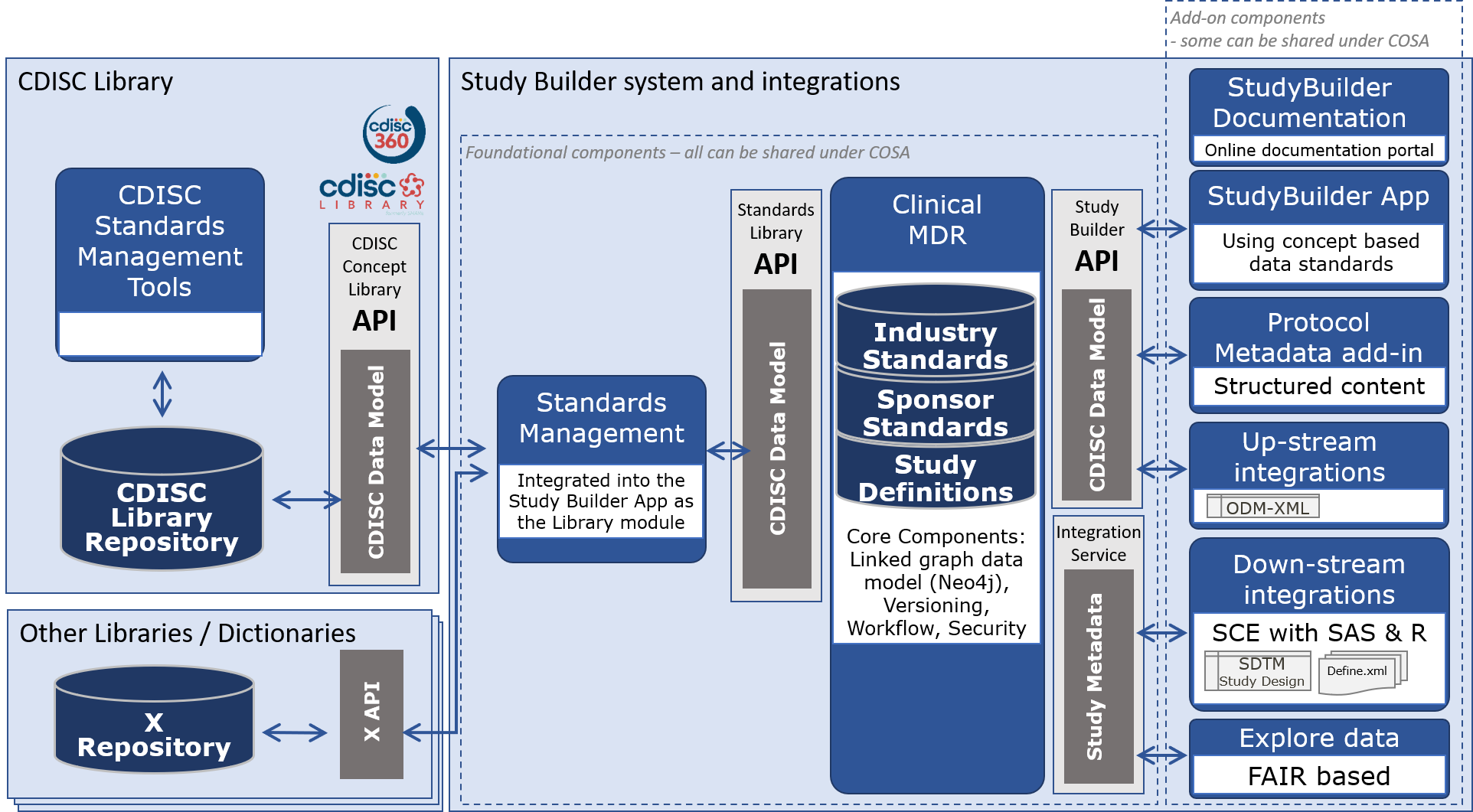
# Conceptual Architecture
This section describes the conceptual architecture for OpenStudyBuilder and Clinical-MDR with upstream and downstream systems.
- OpenStudyBuilder Documentation Online documentation for the OpenStudyBuilder solution including introduction, user guides, system documentation and data model documentation.
- OpenStudyBuilder App Vuetify based Web application with the UI for creating the study definition specification.
- Protocol Metadata add-in Microsoft Word add-in tool holding the Protocol Template and import features of the structured study specification metadata that relates to the protocol content.
- Up-stream integrations Integrations to upstream clinical systems like CTMS, Trial Supplies, EDC, Study Registries, etc.
- Down-stream integrations Integrations to downstream clinical data systems for SDTM, ADaM, analysis and reporting.
- Explore data FAIR based study search and explore tool utilizing the OpenStudyBuilder metadata with reference to systems holding study data.
- OpenStudyBuilder API and Standards Library API Python based web application based on FastAPI framework supporting all CRUD actions to the database, access control, versioning, workflows and data integrity rules.
- Integration Service Integration to UNIX based Statistical Computing Environment (SCE) with SAS and R.
- Clinical MDR Neo4j linked graph database and data model supporting the library standards, study definitions including fine granularity of versioning, audit trail, workflows and access control.
- Standards Management Integrated into the OpenStudyBuilder App as the Library module managing concepts, dictionaries, code lists, syntax templates, project and TA standards.